-
Regulatory Requirements
According to Article 37 of “Food Safety Law of the People's Republic of China”: Safety assessment documents on the relevant product shall be submitted to the health administrative department of the State Council for the production of food with new food raw materials or for the production of a new variety of food additive or food-related products. Within 60 days of receipt of an application, the authority shall organize a review and, if the food safety requirements are satisfied, decide to grant a permit and announce it to the public or, if the food safety requirements are not satisfied, decide not to grant a permit, and provide a written explanation of the reasons for the decision.
Competent department
National Health Commission of the People’s Republic of China (The former National Health and Family Planning Commission NHFPC)
Relevant laws and regulations
“Food Safety Law of the People's Republic of China”
“Administrative Measures for the Safety Review of New Food Raw Materials”
“Declaration and Acceptance Regulations of New Food Raw Materials”
“New Food Raw Materials Safety Review Procedures”
Declaration range
New food raw materials shall have the quality of food raw materials, satisfy necessary nutritional requirements, be non-toxic and harmless and pose no acute, subacute, chronic or other potential harm to human health.
The term “new food raw materials” refers to the following items which are not of traditional eating habits in China:(1) Animals, plants and microorganisms.
(2) Ingredients extracted from animals, plants and microorganisms.
(3) Food ingredients the original composition of which has been changed.
(4) Other newly developed food raw materials.
Application materials(1) Application form.
(2) Research and development report on the new food raw material.
(3) Safety evaluation report.
(4) Production techniques.
(5) Relevant implemented standards applied to the material (including safety requirements, quality specifications and inspection measures).
(6) Label and manual.
(7) Information on utilization thereof and domestic and international research thereon as well as relevant safety evaluation materials.
(8) Power of attorney for declaration (provided when entrusting agent to do the declaration);
(8) Other materials conducive to evaluation and review.
When applying for import of a new food raw material, in addition to materials described above, the applicant shall also submit the following materials:(1) Supporting documents issued by relevant authority or institution in the exporting country (region) allowing such product to be produced or sold in the exporting country (region).
(2) Supporting documents for review or certification of the manufacturer issued by relevant institution or organization in the country (region) where the manufacturer is located.
Declaration Procedure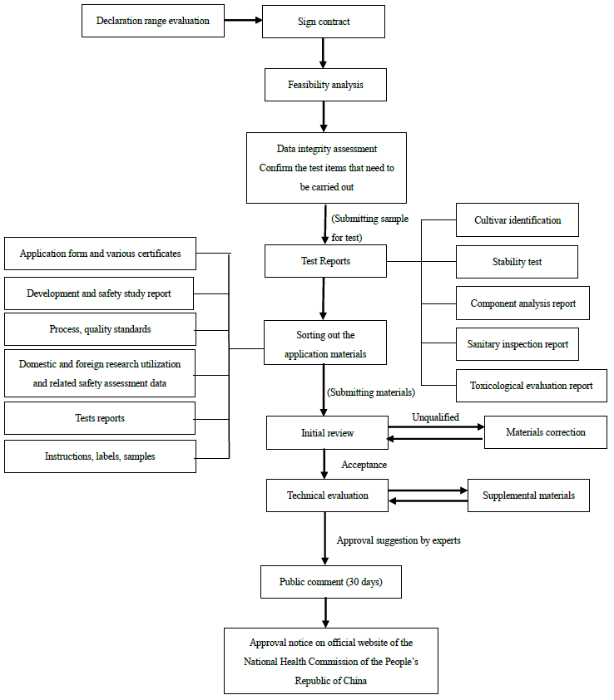
Our services- Regulatory advice;
- Product declaration feasibility analysis;
- Guide and assist enterprise to prepare the required materials for declaration;
- Arrange and coordinate relevant tests;
- Evaluate, review, collate, submit and provide professional application advice for the application materials;
- Participate in technical review meetings and conduct on-site defenses;
- After the review, assist the enterprise to prepare and submit the correction materials;
- Follow up the review process, report progress to the customer in a timely manner, and help customers solve problems that may be encountered during the declaration process.







