-
Cosmetics Administrative Licensing refers to administrative approval for the use of new cosmetic ingredient, the production of domestic special functional cosmetics product, and the first import of cosmetics product. All imported cosmetics, including special and non-special, must not be sold until approved by the State Food and Drug Administration prior to sale.
-
Notification type
● New cosmetic ingredient administrative licensing notification
● Domestic special functional cosmetics product administrative licensing notification
● Domestic non-special functional cosmetics product record certificate application
● Import of non-special functional cosmetics record certificate application
● Import of special functional cosmetics administrative licensing notificationNote: The cosmetic ingredient or cosmetics product with name different from any notified one, without record certificate or administrative license, must be notified.
-
Cosmetics Notification Process
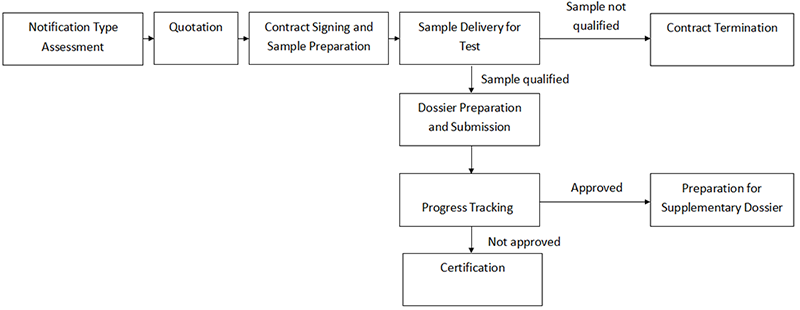
-
Format Requirements for Notification Dossier
1.Original dossier and 4 copies for the first time application of special functional cosmetics product administrative licensing notification; (Copies should be clear and consistent with the original.);
2.Original dossier for application of record certificate, extension, updating, or approval reissuing;
3.Stamps in every pages or stamp across pages in original notification dossier except inspection reports, notary document, official certificates and third party certificates;
4.Printed in books of A4 format, with clear distinguishing marks and provisioned sequence;
5.With Chinese legal units of measurement;
6.Clear and complete notification dossier, in which the descriptions of the same item should be consistent;
7.Chinese translations in front of non-Chinese parts, except oversea address, website, registered trademark, patent name, SPF, PFA/PA, UVA, UVB, and etc., for which non-Chinese language must be used;
8.Paper documents and electronic copies of product formulations;
9.Paper documents and electronic copies should be consistent.
-
Dossier Requirements for New Cosmetic Ingredient Administrative Licensing Notification:
(A)Application Form for New Cosmetic Ingredient Administrative Licensing;
(B)R&D Report
(1)Background, process and related technical information on ingredient R&D;
(2)Source, physical and chemical properties, chemical structure, molecular formula, and molecular weight of ingredient;
(3)Purpose, basis, scope and limit of ingredient use.(C)Descriptions and diagrams of the production process;
(D)Ingredient quality and safety control requirements, including specifications, test methods, substance with potential risk and its risk management measures;
(E)Data for toxicological safety evaluation, including substance with potential risk in ingredient;
(F)Stamped copies of authorization document, and business license for authorized agent in notification;
(G)Other information helpful in administrative licensing notification.
A sample should be provided for inspection.
-
Dossier Requirements for imported non-special functional cosmetics record notification:
(A)Administrative licensing application form for imported non-special functional cosmetics record notification;
(B)Name basis for product Chinese name;
(C)Product formulation;
(D)The product quality and safety control requirements;
(E)Original product packaging (including product label, and manual); Packaging design for China market (including product label, and manual);
(F)Inspection report issued by inspection agency authrized by the State Food and Drug Administration, and related information;
(G)Safety assessment data for the potential risk in product;
(H)Stamped copies of authorization document of recorded administrative license, and business license;
(I)Commitment for compliance of ingredient and source of ingredient with prohibition or restriction of substance with high risk in BSE area;
(J)Sales Certificate in country/region of production or country/region of origin;
(K)Other information helpful in record notification.
A sales sample sealed by Authorized Inspection Institute should be provided.
-
Dossier Requirements for imported special functional cosmetics administrative licensing notification:
(A)Administrative licensing application form for imported special functional cosmetics record notification;
(B)Name basis for product Chinese name;
(C)Product formulation;
(D)Descriptions and diagrams of the production process;
(E)The product quality and safety control requirements;
(F)Original product packaging (including product label, and manual); Packaging design for China market (including product label, and manual);
(G)Inspection report issued by inspection agency authorized by the State Food and Drug Administration, and related information;
(H)Safety assessment data for the potential risk in product;
(I)Active ingredients and related scientific literatures, for application of hair tonic, body building, breast care cosmetics product;
(J)Stamped copies of authorization document of recorded administrative license, and business license;
(K)Commitment for compliance of ingredient and source of ingredient with prohibition or restriction of substance with high risk in BSE area;
(L)Sales Certificate in country/region of production or country/region of origin;
(M)Other information helpful in record notification.
A sales sample sealed by Authorized Inspection Agency should be provided.
-
Dossier Requirements for domestic special functional cosmetics administrative licensing notification:
(A)Administrative licensing application form for domestic special functional cosmetics record notification;
(B)Name basis for product Chinese name;
(C)The product quality and safety control requirements;
(D)Original product packaging (including product label, and manual);
(E)Inspection report issued by inspection agency authorized by the State Food and Drug Administration, and related information;
(F)Safety assessment data for the potential risk in product;
(G)Audit opinion on hygiene condition in production issued by provincial food and drug administration
(H)Active ingredients and related scientific literatures, for application of hair tonic, body building, breast care cosmetics product;
(I)Other information helpful in record notification.
A sales sample sealed by Authorized Inspection Agency should be provided.
-
Our Services
●Regulations consulting;
●Regulatory compliance assessments;
●Guidance and assistance for notifier to prepare dossiers;
●Translation of non-Chinese documents;
●Test arrangements and communications;
●Data evaluation, review, preparation and submission;
●Supplementary information resubmission after expert review meeting
●Communication, coordination, and trouble-shooting in notification.







